
The Standard English unit is pounds mass per cubic foot ( lbm/ft 3).Įlectron Affinity and Electronegativity of KryptonĮlectron Affinity of Krypton is - kJ/mol. The standard SI unit is kilograms per cubic meter ( kg/m 3). In other words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. It is an intensive property, which is mathematically defined as mass divided by volume: Typical densities of various substances at atmospheric pressure.ĭensity is defined as the mass per unit volume. For 63Cu, the atomic mass is less than 63, so this must be the dominant factor. A nucleus with greater binding energy has lower total energy, and therefore a lower mass according to Einstein’s mass-energy equivalence relation E = mc 2.

Krypton – Properties Element Krypton Atomic Number 36 Symbol Kr Element Category Noble Gas Phase at STP Gas Atomic Mass 83.798 Density at STP 3.75 Electron Configuration 3d10 4s2 4p6 Possible Oxidation States 0 Electron Affinity - Electronegativity 3 1st Ionization Energy 13.9996 Year of Discovery 1898 Discoverer Ramsay, Sir William & Travers, Morris Thermal properties Melting Point -157.36 Boiling Point -153.22 Thermal Conductivity 0.00949 Specific Heat 0.248 Heat of Fusion 1.638 Heat of Vaporization 9.029 A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is a member of group 18 (noble gases) elements.
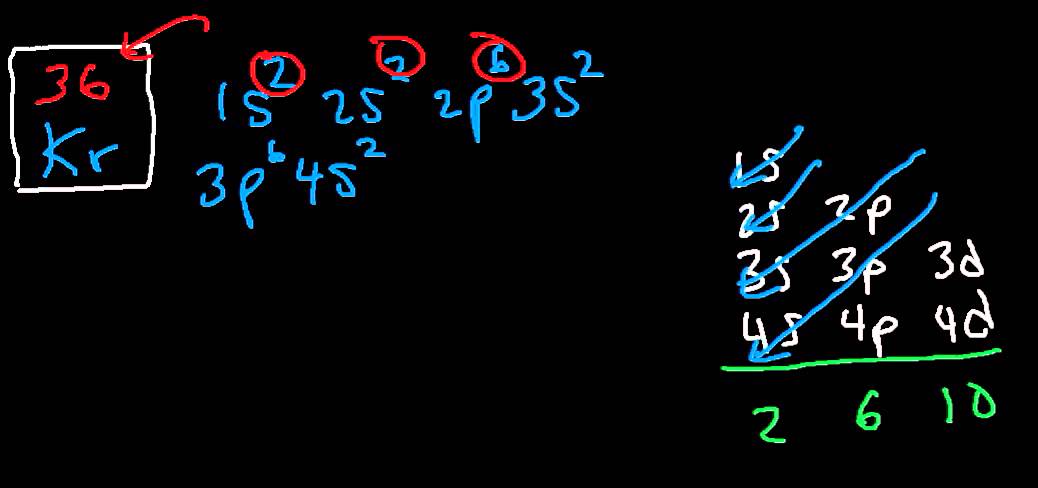
Krypton is a chemical element with atomic number 36 which means there are 36 protons and 36 electrons in the atomic structure.


 0 kommentar(er)
0 kommentar(er)
